SIMICA publications available for download!
30) J Infect Dis 2023; just accepted. DOI: 10.1093/infdis/jiad135.
29) J Am Chem Soc 2023;145(19):10790-10799. DOI: 10.1021/jacs.3c01960.

28) ACS Pharmacol Transl Sci 2022;5(11):1156-1168. DOI:10.1021/acsptsci.2c00163.
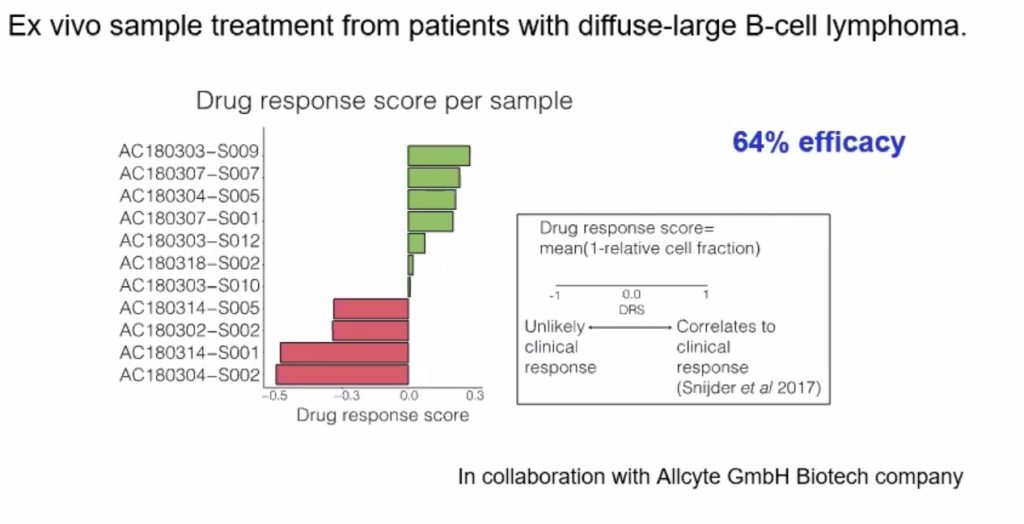
27) Cancers 2022;14(11):2724. DOI: 10.3390/cancers14112724.
26) Pharmaceutics 2022;14(7):1351. DOI: 10.3390/pharmaceutics14071351.
25) Gels 2022;8(8):488. DOI: 10.3390/gels8080488.
24) Mol Biomed 2022;3(1):26. DOI: 10.1186/s43556-022-00086-z.
23) Mikrochim Acta 2022;189(10):368. DOI: 10.1007/s00604-022-05441-z.
22) Pharmaceutics 2022;14(4):840. DOI: 10.3390/pharmaceutics14040840.
21) Biomedicines 2022;10(5):1070. DOI: 10.3390/biomedicines10051070.
20) Int J Mol Sci 2022;23(8):4240. DOI: 10.3390/ijms23084240.
19) Int J Mol Sci 2021;22(10):5217. DOI: 10.3390/ijms22105217.
18) Mol Imaging Biol 2023;25(1):122-132. DOI: 10.1007/s11307-021-01657-2.
17) Pharmaceutics 2021;13(8):1321. DOI: 10.3390/pharmaceutics13081321.
16) Pharmaceutics 2021;13(10):1670. DOI: 10.3390/pharmaceutics13101670.
15) Int J Mol Sci 2021;22(11):5730. DOI: 10.3390/ijms22115730.
14) Bioconjug Chem 2021;32(8):1812-1822. DOI: 10.1021/acs.bioconjchem.1c00276.
Interested on chemically triggered IEDDA reactions for protein modification? On this publication we found that the reactivity of alkyne-BF3 dienophiles can be activated by Lewis acids to fast react with tetrazines.

13) Bioconjug Chem 2021;32(1):121-132. DOI: 10.1021/acs.bioconjchem.0c00551.
Check out our recent paper. We synthesized and evaluated new carbonyl acrylate reagents for cysteine modification having Diels-Alder handles for in vivo pretargeting applications.
This paper was highlighted on a virtual issue on “Bioorthogonal Chemistry and Bioconjugation” at Bioconjugate Chemistry.
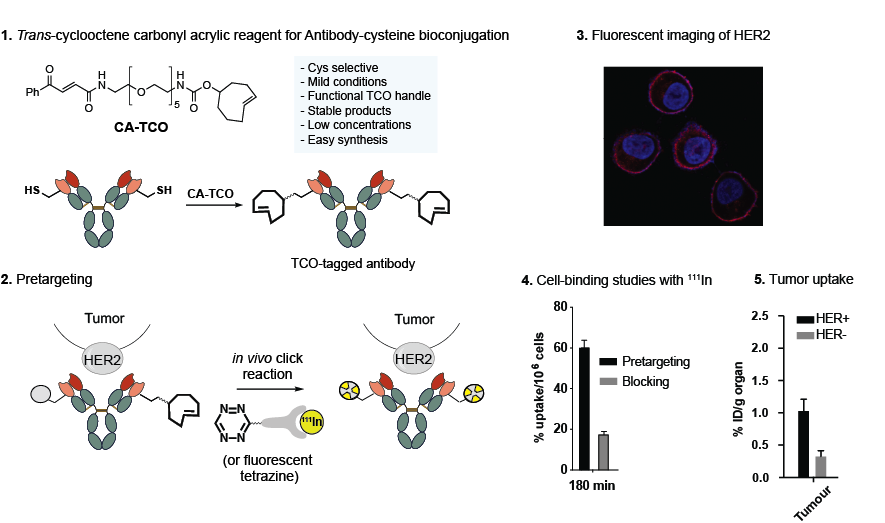
12) Biomaterials 2021;268:120580. DOI: 10.1016/j.biomaterials.2020.120580.
11) Br J Pharmacol 2021;178(11):2284-2304. DOI: 10.1111/bph.15373.
10) Molecules 2021;26(11):3272. DOI: 10.3390/molecules26113272.
9) J Control Release 2020;323:1-11. DOI: 10.1016/j.jconrel.2020.03.050.
8) Pharmaceutics 2020;12(11):1054. DOI: 10.3390/pharmaceutics12111054.
7) Pharmaceutics 2020;12(11):1022. DOI: 10.3390/pharmaceutics12111022.
6) Front Chem 2020;8:496. DOI: 10.3389/fchem.2020.00496.
5) J Nanopart Res 2020;22:115 DOI: 10.1007/s11051-020-04832-8
4) Pharmaceutics 2020;12(11):1054. DOI: 10.3390/pharmaceutics12111054.
3) Molecules 2020;25(5):1181. DOI: 10.3390/molecules25051181.
2) Molecules 2020;25(9):2219. DOI: 10.3390/molecules25092219.
1) J Am Chem Soc 2020;142(24):10869. DOI: 10.1021/jacs.0c01622